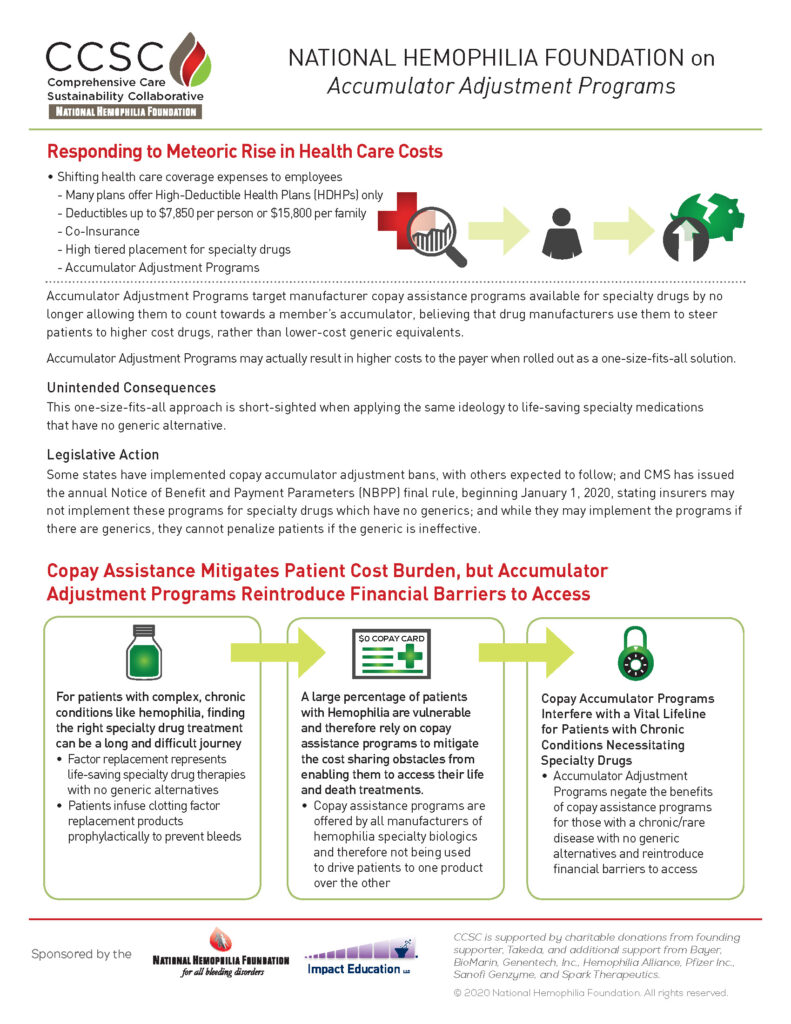
Advocacy
Alone we can do so little; Together we can do so much. -Helen Keller
The Lone Star Bleeding Disorders Foundation is a member of the Texas Bleeding Disorders Advocacy Coalition. We advocate at the state and federal level on issues important to the bleeding disorders community. The Coalition is a joint effort of the Lone Star Bleeding Disorders Foundation and Texas Central Hemophilia Association. Learn more about the coalition at txbdcoalition.org.
2023 Austin Days!
Join us for our 2023 Austin Days, March 1-2! Please send your completed registration form to Karla by January 10th, 2023! Download the registration form here.
2023 NHF Washington Days!
NHF Washington Days is back in person, March 8-10, 2023. LSBDF and the Coalition will be sending a limited number of people to participate. Applications are now available here.
Legislative Priorities